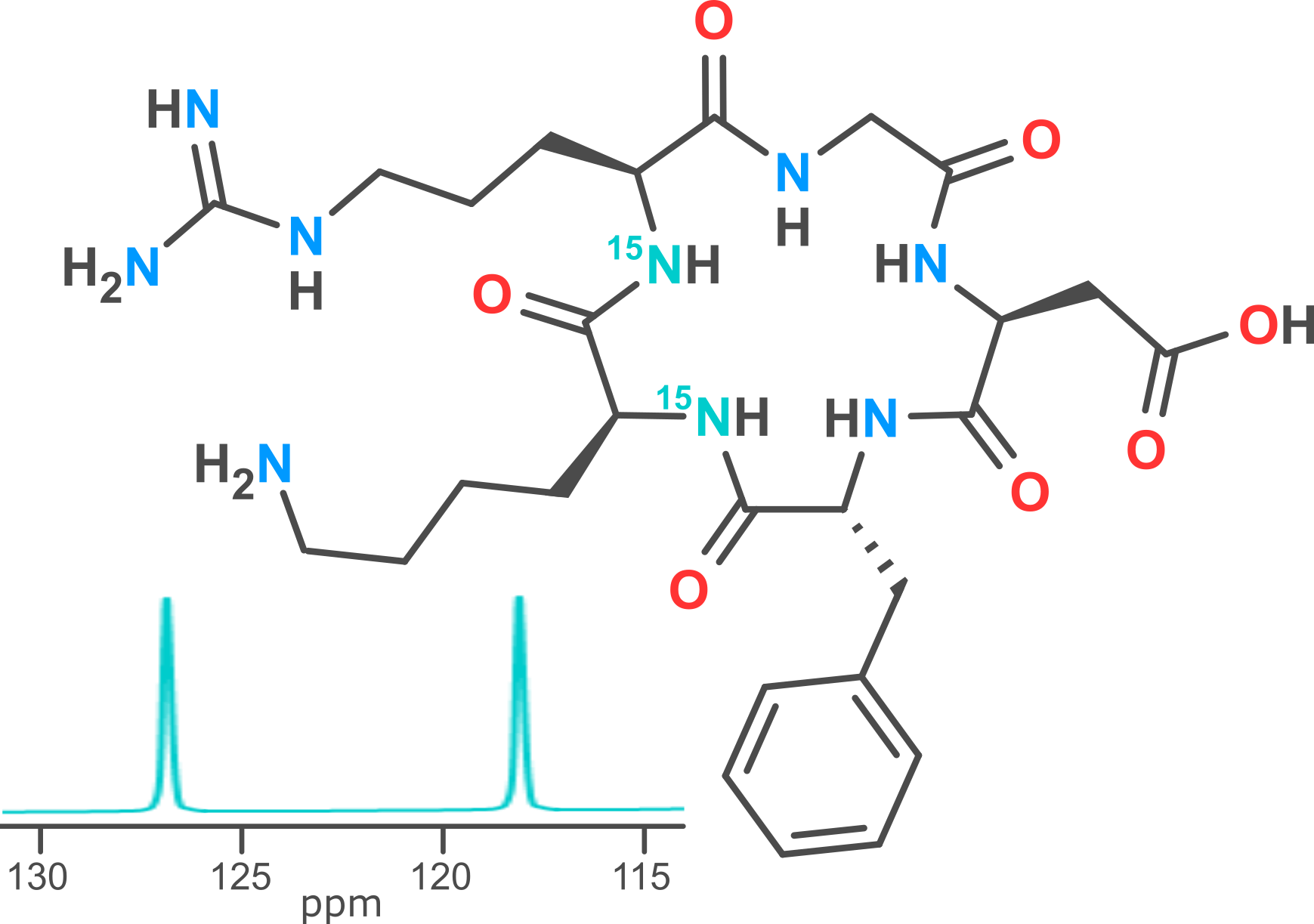
Site-specific labelling of peptides and proteins with stable NMR-active nuclei (such as deuterium, carbon-13 and nitrogen-15) is a valuable strategy for measuring atomic distances and identifying the chemical shift of a particular residue. This approach can be used to study molecular dynamics, ligand-receptor interactions and aid in the elucidation of complex tertiary structures. For applications such as these, we recommend that NMR spectroscopists use peptides and proteins isolated as the hydrochloride salt (instead of trifluoroacetate), particularly for fluorine-19 experiments. Modpep can supply tens of milligrams of isotopically labelled material at purities in excess of 95%.